Written by Lucie Keunen
Product Owner Andaman7
Android 2.5 - iOS 2.5
Description
Patients using Andaman7 in a clinical trial now have access to a motivating dashboard that gives an overview of the study protocol. They can thus have a clear view of past, current and future tasks.
Why is it important?
The success of a clinical study depends on various factors. Among these, patient retention is a significant challenge. As a matter of fact, a high rate of dropouts can have serious consequences; it may compromise the validity of the results or cause costly delays.
It is therefore important to fight against the causes of dropout. The digitalization of the studies and the dashboard implemented by Andaman7 work on several aspects:
- Clear and precise expectations - the patient knows exactly what to expect when they choose to participate in the study. He/she clearly sees the steps that have already been completed and those still to come.
- Reminders and notifications - the patient does not lose sight of the study and the actions he/she has to take, even in the long term.
- Near real-time synchronization – the researchers can quickly learn about, and act accordingly, on any side effects, adverse events or out of range biometrics reported by the patient.
- Pleasant interface - the patient will be seeing his/her progress in real time in a visually appealing dashboard, something which will motivate him/her to complete the questionnaires, study visits, and other tasks.
- Patient empowerment - the patient always has access to the data he/she provides for the study, which in turn enriches his/her health record.
- Reduce workload for patients and study sites - the patient does not have to always go to the study site, and the collected data no longer needs to be manually transcribed.
How does it works?
The dashboard offers a simple and clear view of the entire study. The questionnaires, presented in chronological order in the form of cards, can have three states:
- Completed
- To be filled
- Blocked (to be completed in the future)
This allows the patient to see at a glance, where he/she appears in the course of the study; what path has already been covered, what the deadlines are, and when the next actions to be done are planned.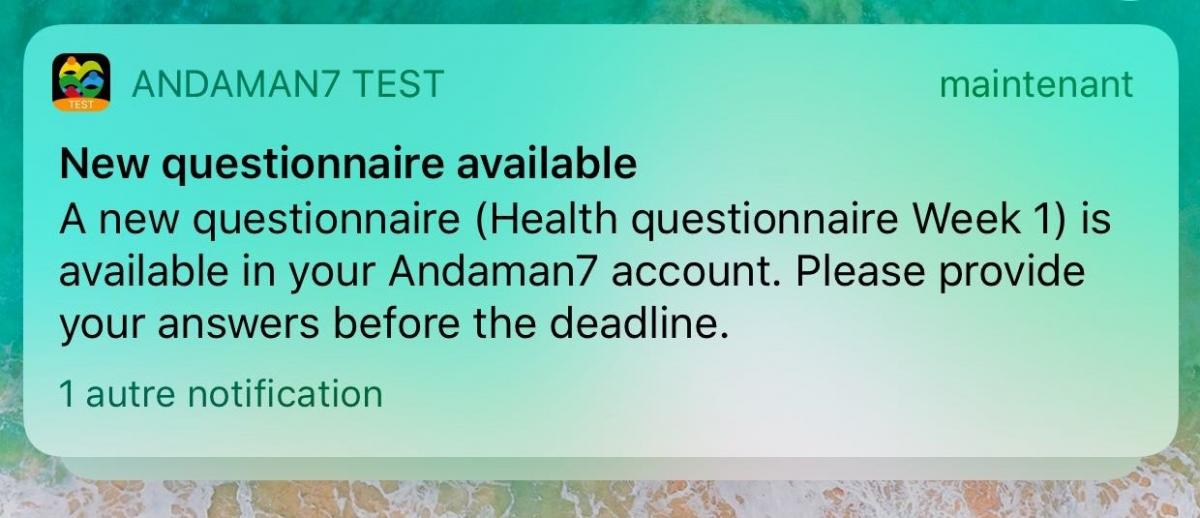
A notification is sent to the patient, whenever he/she has to fill out a new questionnaire. The actions and questionnaires to be completed are put in the foreground of the dashboard, enabling the quick identification of things to be completed. The progression indicators motivate the patient to move forward and to avoid forgetting to answer certain questions.
As an option, some actions can also be initiated by the patients themselves; such as, the notification of side effects or the occurrence of a significant event.
The encoded data history is always accessible and searchable, and the results of the study can eventually be transmitted to the patient.
Where can we see it?
The dashboard is available for any clinical study that is conducted in partnership with Andaman7.







